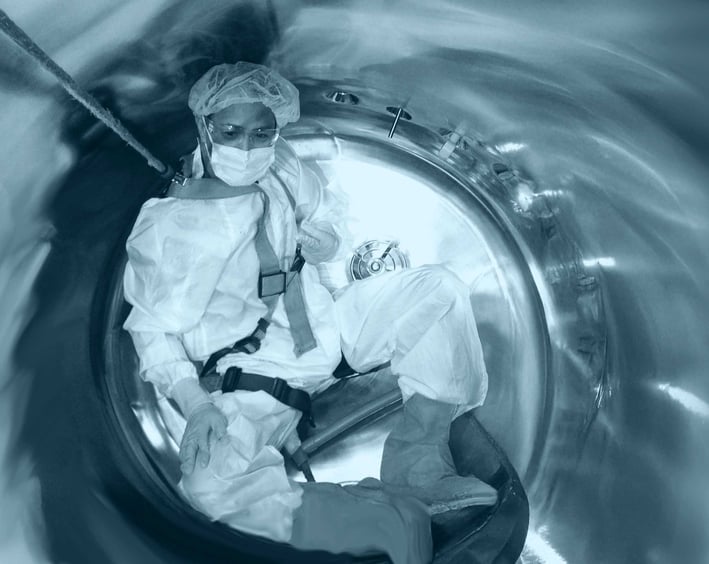
ValCom Consulting brings trusted leadership and deep expertise to support organizations across the biotechnology industry.
From emerging clinical-stage cell and gene therapy firms to leading pharmaceutical manufacturers, our founding members bring over 50 years combined experience to the biotechnology industry.
Quality Engineering /
Quality Assurance ValidationQuality Systems Support
(CAPA, Change Control, Deviations)Supplier Quality Management
Batch Record Review
Audit Readiness – Audit Support
Risk Evaluations and Gap Assessment
Get your project done fast.
Find out how ValCom Consulting can support your success.
Air Flow Visualization /
Environmental Monitoring Performance Qualification (EMPQ) Facilitation and SupportFUE Design and Management
Annex 1 Readiness
Commissioning, Qualification, and
Validation SupportValidation Maintenance
Analytical Instrumentation Qualification
Computer System Validation Support
Facility and Utilities Verification
FAT / SAT Support
Enterprise System Planning and Implementation
SOP and Document Writing for CGMP
Building Automation System Commissioning and Qualification


QUALITY


CQV
MANAGEMENT


Overall Project Management to Support the Engineering Lifecycle
(Design to Facility Fit and Finish)Facility / Suite / Laboratory Expansion
Tech Transfer
Consent Decree Remediation